
UCF Clinical SAS® Internship Program
In a Nutshell
The clinical research industry is a fast-growing field that uses innovative data science and biostatistics to help save lives. In our Clinical SAS® Internship Program, developed specifically for the University of Central Florida (UCF) students and graduates, you will learn modern statistical programming tools used to analyze clinical trial data – and get paid! By the end of the program, you will:
- Have a jumpstart on a successful career in data science
- Understand statistical methods for clinical trial analyses
- Have insight into pharmaceutical industry processes and standards
- Be able to perform statistical analysis of clinical data using SAS® analytics software
- Improve your communication, presentation, and collaboration skills (all musts for success in the world of data science!)
Our Mission
To provide leadership while offering students exposure to desirable skill sets in theory, practice methods, and applications of biostatistics.
Paid Part-Time Clinical SAS® Internship
3-month commitment, 20 hours per week
Learn Clinical SAS® Analytics Software
SAS/Base, SAS/STAT, SAS/Graph, SQL
Full-time Employment at Intego Clinical
For top-performing interns with recommendation
Work on Top-Priority Clinical Studies
Help save lives and improve processes
How To Apply
Join a global biometrics CRO company focused on the future of statistical analysis of clinical trials
Ideal candidates for this program are students or graduates majoring in Applied Mathematics, Statistics or Data Science, but the program is open for anyone with a strong mathematical background. Up to 10 internships are available
If accepted, you’re officially on your way to a lucrative career in data science!
Internships start mid-May.
Career Opportunities
Statistical Programmer Analysts are professionals who apply mathematical skills and statistical software to comprehensive data analysis in medicine, biology, life sciences and many other related fields.
Clinical Research Organizations
CROs
Pharmaceutical Companies
Worldwide
SAS® Analytics Opportunities
In banking, finance, retail, etc.
Intego Clinical
For top-performing interns
Internship Program Format
Internships are part-time, paid positions that run for three months. Expect a 20 hour per week workload. Top-performing interns will be offered a full-time position at Intego Clinical.
Interns will participate in online and in-person training sessions. To encourage collaboration and improve communication skills, some sessions will involve group exercises. Students will be divided into separate teams, and each team will be mentored by a representative from Intego Clinical who will share industry-specific knowledge and project development advice.
Sessions & Trainings
This course is designed to teach students how to execute SAS® programs in a Microsoft Windows environment. It provides an overview of the SAS® system under Microsoft Windows and a fundamental grounding in the following: SAS® DATA step, basic data manipulation including reading raw data into SAS®, using formats and informants, functions, conditional processing, subsetting and joining data sets. The course is also designed to cover the basic reporting procedures, including PROC Print, PROC Freq, PROC Means, PROC Tabulate and PROC Report, as well as the output delivery system (ODS), SQL, SAS®/GRAPH, macros, arrays and other Base SAS® procedures. This course provides a solid grounding in the more advanced features of SAS®.
This course acts as an introduction to the fundamental concepts and approaches that govern the design, analysis and interpretation of clinical research studies. It provides a basic overview of the clinical research industry and new drug development. Students will be introduced to the language that is typically associated with clinical research and the pharmaceutical industry. They will have an opportunity to learn, understand and apply this terminology in the context of clinical research. This course also covers an overview of the process of new drug development from discovery through regulatory to approval and introduction to the market. It’s designed to provide students with the background needed to pursue a number of additional courses in the areas of biostatistics, clinical data analysis and biostatistical programming.
This course is highly relevant for modeling and data analysis in many areas, including medicine, actuarial science, economics and other social sciences. Statistics II covers classic repeated measures model, random effect models, generalized estimating equations (GEEs), hierarchical models and transitional models for binary data, marginal vs. mixed effects models, model fitting, model checking, clustering and implications for study design. The module also includes discussion of missing data techniques, Bayesian and likelihood methods for GLMs and various fitting algorithms such as maximum likelihood and generalized least squares. This course also provides an introduction to methods for time-to-event data with censoring mechanisms. Topics include life tables, nonparametric approaches (e.g., Kaplan-Meir, log-rank), semi-parametric approaches (e.g., Cox model), competing risks (introduction to Poisson regression as connection to the Cox model) and time-dependent covariates. Additionally, the methodology and rationale for Bayesian methods and their applications will be briefly discussed.
This course is designed to be very hands-on and focused on the practical aspects of performing clinical trial analyses in the pharmaceutical industry. Skills will include creating datasets, tables and listings, preparing graphics, and performing commonly used statistical analysis. Important topics such as CDISC, MedDRA, WHODrug and SOPs, and other industry regulations and standards, will also be presented to the students during the course. During the workshops, students will import and export raw data files, manipulate and transform data, combine SAS® data sets, perform analysis, create basic detail and summary reports using SAS® procedures, identify and correct data, syntax and programming logic errors and use output delivery systems. Health-related data sets will be provided for students to use.
The data interpretation module is designed to help students who have no background in life sciences prepare for data interpretation assessments in clinical practice. It explores a number of key topics in medicine. Each topic is set around an image or investigation, such as vital signs, an X-ray, CT scan or blood film, that is used to test identification and interpretation of the data provided. Through explanation of the correct and incorrect answers, students can learn from their mistakes in a safe setting. This course aims to teach students how to interpret clinical data and the outputs generated during the Clinical SAS® Programming module.
Apply Now for The UCF Clinical SAS® Internship Program
Use your math and statistical training on real-life projects. Help save lives through top-priority clinical studies.
Intego Clinical is a proud sponsor of UCF’s Big Data Analytics Symposium
Learn more about our Clinical SAS Internship Program at our booth or info session at UCF’s next Big Data Analytics Symposium!

Big Data Analytics Symposium 2026
March 23, 2026
Frequently Asked Questions
General Questions
Currently, there are up to ten internship opportunities available with Intego Clinical.
This internship starts in mid-May and lasts for three months. It’s part-time with an expected workload of 20 hours per week.
This is a part-time internship with an expected workload of 20 hours per week.
Yes, this internship is paid.
Each intern that completes the full internship program will receive a Certificate of Completion from Intego Clinical to add to their resumes. Intego Clinical can also provide letters of recommendation for interns who do not transition into full-time employment with Intego Clinical.
No problem! Pharmaceutical applications are just part of the program. If you decide that the pharma industry isn’t for you, you will still gain a deep knowledge of SAS® and statistical analysis that will open doors for you in other industries, such as finance and banking, retail and logistics, and many others. Intego Clinical can provide a letter of recommendation for interns who complete the internship program and choose to pursue a career elsewhere.
SAS® is internationally regarded and sets the industry standard in clinical trial analysis. It is also the only product that has been officially approved by the FDA as a format for clinical data. You can find more information about the applications for SAS® in life science and other industries by clicking here.
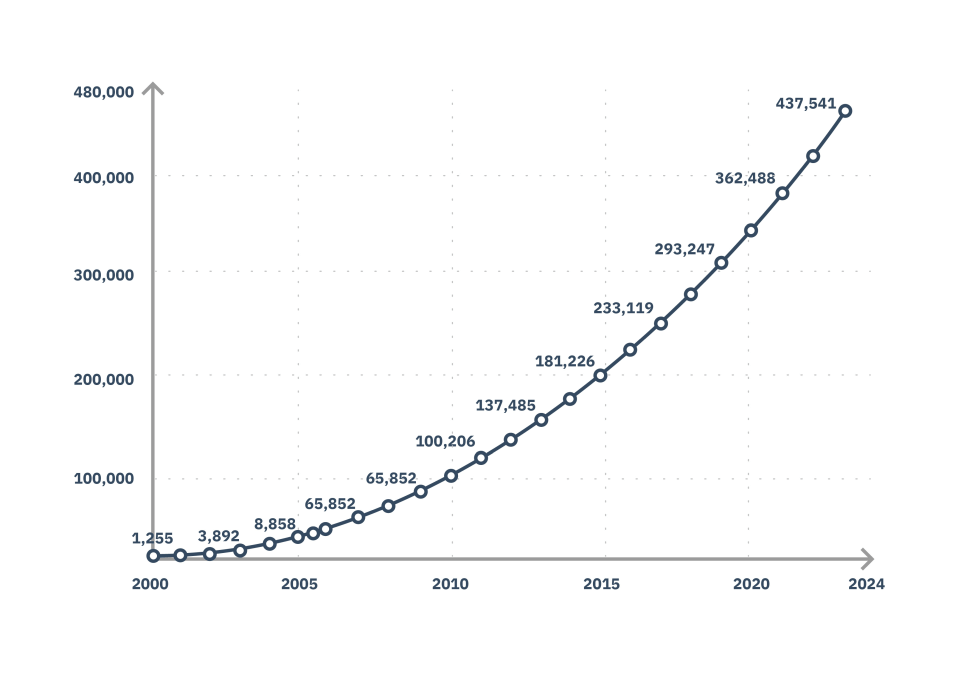
Increase in Demand for Professional Biostatisticians
The number of clinical trials worldwide has grown exponentially, resulting in a high demand for biostatisticians, statistical programmer analysts, clinical data managers and other professionals in the industry.
Number of clinical studies performed worldwide: 10 thousand in 2006 to over 500 thousand in 2025.
Source: clinicaltrials.gov
Apply Now for The UCF Clinical SAS® Internship Program
Use your math and statistical training on real-life projects. Help save lives through top-priority clinical studies.
All applications due before April 15, 2026
