
Biostatistics Certificate Program

In A Nutshell
Biostatistics integrates the medical field with math, statistics, and life science to drive clinical research and new drug development. In our certificate program at Karazin National University, students explore modern statistical and programming tools used for analyzing clinical trial data in order to:
- Jumpstart a successful career in data science
- Gain an understanding of statistical methods for clinical trial analysis
- Learn pharmaceutical industry aims and standards
- Perform statistical analysis of clinical data using SAS® analytics software
- Improve communication, presentation and collaboration skills (All musts for success in the world of data science!)
Program Partners
Senior professionals. Subject matter experts. Industry leaders.
Over 90% employment rate
For program graduates
Increase in Demand for Professionals
High demand for biostatisticians and statistical programmer analysts
Paid Internship for top-performers
Cost fully covered by Intego Clinical for top-performing students
SAS® Academic Partnership
Opens doors to careers in industries like banking, finance, retails, etc.
Admissions Criteria
Jumpstart your career. Get started on your Biostatistics Certificate today.
This program is offered exclusively in Ukraine.
If approved, you’re officially a student of The Center for Biostatistical Programming and on your way to a lucrative career in data science!
Career Opportunities
Biostatisticians and statistical programmer analysts are professionals who apply mathematical skills and statistical analysis to comprehensive research in medicine, biology, life sciences and many other related fields.
Clinical Research Organizations
Including Intego Clinical
Pharmaceutical Companies
Worldwide
SAS® Analytics Opportunities
In banking, finance, retail, etc.
Program Format
All classes are held remotely with flexible time integrated into university schedules, including some evening lessons. To ensure success in the program, students must dedicate at least 15–18 hours a week over a fall and spring semester to participate.
Many classes and guest speaker sessions are conducted in English. All educational materials are available in English only.
To encourage collaboration and improve communication skills, some courses involve group exercises. Students will be divided into separate teams, and each team will be mentored by a representative from Intego Clinical who will share industry-specific knowledge and project development advice.

Program Curriculum
Fall Semester
This course is designed to teach students how to execute SAS® programs in a Microsoft Windows environment. It provides an overview of the SAS® system under Microsoft Windows and a fundamental grounding in the following: SAS® DATA step, basic data manipulation including reading raw data into SAS®, using formats and informants, functions, conditional processing, subsetting and joining data sets. The course is also designed to cover the basic reporting procedures, including PROC Print, PROC Freq, PROC Means, PROC Tabulate and PROC Report, as well as the output delivery system (ODS), SQL, SAS®/GRAPH, macros, arrays and other Base SAS® procedures. This course provides a solid grounding in the more advanced features of SAS®.
This course acts as an introduction to the fundamental concepts and approaches that govern the design, analysis and interpretation of clinical research studies. It provides a basic overview of the clinical research industry and new drug development. Students will be introduced to the language that is typically associated with clinical research and the pharmaceutical industry. They will have an opportunity to learn, understand and apply this terminology in the context of clinical research. This course also covers an overview of the process of new drug development from discovery through regulatory to approval and introduction to the market. It’s designed to provide students with the background needed to pursue a number of additional courses in the areas of biostatistics, clinical data analysis and biostatistical programming.
This course is designed to prepare students to communicate effectively in English within the pharmaceutical industry. This course will equip learners with the linguistic skills and special vocabulary required to understand daily situations in the target work environment. A variety of engaging topics and motivating role-plays, as well as extensive practical exercises, provide our students with an understanding of how to communicate effectively in different areas of the pharmaceutical industry.
This course provides foundations of database systems, focusing on basics such as relational algebra and data modeling, schema normalization, query optimization and transactions. It teaches the algebraic query language that forms the formal foundations of SQL as well as SQL advanced features. It is designed for students who have no prior database experience, although students who have taken an undergraduate course in databases are encouraged to attend.
This course explores the collection, analysis and interpretation of data. It develops an understanding of the appropriateness of different methods of data collection, with a particular focus on the methods used to sample data from a population. Students will be taught to recognize and provide examples of the different types of data that arise in public health and clinical studies, interpret differences in data distributions via visual displays, calculate standard normal scores and resulting probabilities and calculate and interpret confidence intervals for population means and proportions. Students will be exposed to a theoretical framework for linear and generalized models. The course covers the following linear models: multivariate normal theory, least squares estimation, limiting chi-square and F-distributions, sum of squares (partial, sequential) and expected sum of squares, weighted least squares, orthogonality, analysis of variance (ANOVA). The second half of the semester focuses on generalized linear models: binomial, Poisson, multinomial errors, introduction to categorical data analysis, conditional likelihoods, quasi-likelihoods, model checking and matched pair designs.
Spring Semester
This course is designed to be very hands-on and focused on the practical aspects of performing clinical trial analyses in the pharmaceutical industry. Skills will include creating datasets, tables and listings, preparing graphics, and performing commonly used statistical analysis. Important topics such as CDISC, MedDRA, WHODrug and SOPs, and other industry regulations and standards, will also be presented to the students during the course. During the workshops, students will import and export raw data files, manipulate and transform data, combine SAS® data sets, perform analysis, create basic detail and summary reports using SAS® procedures, identify and correct data, syntax and programming logic errors and use output delivery systems. Health-related data sets will be provided for students to use.
The data interpretation module is designed to help students who have no background in life sciences prepare for data interpretation assessments in clinical practice. It explores a number of key topics in medicine. Each topic is set around an image or investigation, such as vital signs, an X-ray, CT scan or blood film, that is used to test identification and interpretation of the data provided. Through explanation of the correct and incorrect answers, students can learn from their mistakes in a safe setting. This course aims to teach students how to interpret clinical data and the outputs generated during the Clinical SAS® Programming module.
This course is designed to prepare students to communicate effectively in English within the pharmaceutical industry. This course will equip learners with the linguistic skills and special vocabulary required to understand daily situations in a work environment. A variety of engaging topics and motivating role-plays, as well as an extensive number of practical exercises, will provide students with an understanding of how to communicate effectively in the different areas of the pharmaceutical industry.
This course is highly relevant for modeling and data analysis in many areas, including medicine, actuarial science, economics and other social sciences. Statistics II covers classic repeated measures model, random effect models, generalized estimating equations (GEEs), hierarchical models and transitional models for binary data, marginal vs. mixed effects models, model fitting, model checking, clustering and implications for study design. The module also includes discussion of missing data techniques, Bayesian and likelihood methods for GLMs and various fitting algorithms such as maximum likelihood and generalized least squares. This course also provides an introduction to methods for time-to-event data with censoring mechanisms. Topics include life tables, nonparametric approaches (e.g., Kaplan-Meir, log-rank), semi-parametric approaches (e.g., Cox model), competing risks (introduction to Poisson regression as connection to the Cox model) and time-dependent covariates. Additionally, the methodology and rationale for Bayesian methods and their applications will be briefly discussed.
This course is designed to prepare students for taking the SAS® Base Programming for SAS® 9 exam. It provides full information about SAS® certification, advantages of certification, typical challenges students will encounter during the exam, an overview of technical aspects and practical experience of mock tests.
Apply Now for The Biostatistics Program
Successful applicants must have a strong mathematical background
Increase in Demand for Clinical Data Professionals
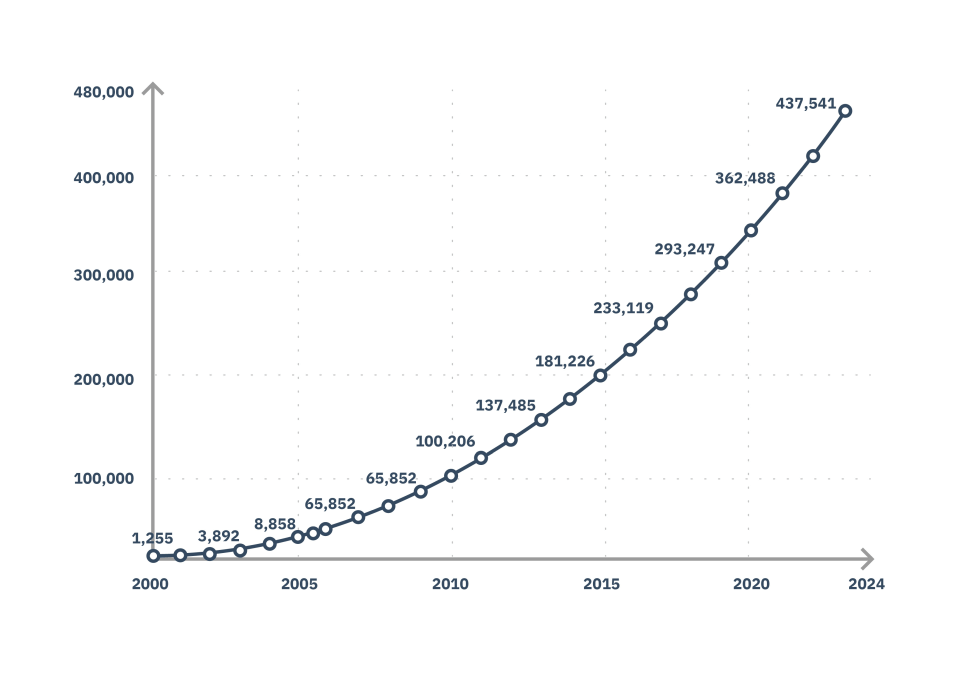
The number of clinical trials worldwide has grown exponentially, resulting in a high demand for biostatisticians, statistical programmer analysts, clinical data managers and other professionals in the industry.
Number of clinical studies performed worldwide: 10 thousand in 2006 to over 500 thousand in 2025.
Source: clinicaltrials.gov
The Center for Biostatistical Programming
The Biostatistics Certificate Program has been running for over a decade without interruption. This center aims to meet the needs of leading pharmaceutical companies and clinical research organizations. Because of its success, what originally started out as a program specifically for graduate students at Karazin’s University School of Mechanics and Mathematics has since opened up to anyone in Ukraine. Complete with mentorships, internships, and employment opportunities, The Center for Biostatistical Programming provides opportunities beyond what you’ll find in any other program.
Frequently Asked Questions
General Questions
With over 35 schools and departments, Karazin National University is one of the oldest and most reputable educational institutions in Ukraine. Our program originally targeted students with a strong background in applied mathematics from the School of Mechanics and Mathematics, but it has since been opened up to anyone in the cities of Kharkiv and Kyiv, Ukraine.
Biostatistics requires knowledge of the pharmaceutical industry and clinical trials; for these courses, we’ve invited professors from the university’s School of Medicine. We’ve also invited instructors from the School of Foreign Languages to improve students’ English language proficiency.
Our program is unique! This is the only biostatistical program in Eastern Europe in which students have the opportunity to both advance their skills in applied statistics and develop expertise in clinical SAS® programming based on real-world projects.
Students will receive a program certificate from Karazin National University and a certificate from The Center for Biostatistical Programming. Those who achieve a passing score of 70% or above on the certification exam will have the opportunity to receive the SAS® Base Programming for SAS® 9 professional certificate from the SAS® Institute.
No problem! Pharmaceutical applications are just part of the program. If you decide that the pharma industry isn’t for you, you will still gain a deep knowledge of SAS® and statistical analysis that will open doors for you in other industries, such as finance and banking, retail and logistics, and many others.
Yes, due to facility restrictions and availability of mentors, the class size for this program is limited to 35 students this year.
SAS® is internationally regarded and sets the industry standard in clinical trial analysis. It is also the only product that has been officially approved by the FDA as a format for clinical data. We have worked hard to foster an academic partnership between Karazin National University and the SAS® Institute. This relationship enables us to offer our students unique opportunities to become experts in the use of industry-standard software. You can find more information about the applications for SAS® in life science and other industries by clicking here.
Application for admission to Fall Semester 2025-26 Biostatistics program
All applications due before September 20, 2025


